Energy is a fundamental concept that relates to just about everything around us, from the warmth of sunlight to the power needed to run our gadgets. One of the essential units of energy is the joule, which is a way to quantify the amount of work done or the energy transferred in a process. Understanding how to calculate joules can be valuable in various applications, such as physics problems, understanding electricity bills, or even gauging the energy content in foods. Let’s explore some methods and tips for calculating joules in different scenarios.
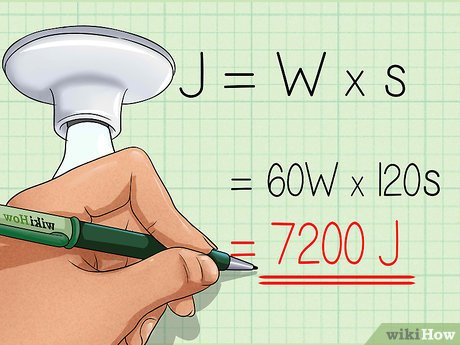
Using Work Equation
The work equation is a basic principle of physics that relates force, distance, and energy. When you apply a force to move an object over a distance, you’re doing work, which is energy transfer.
Detailed Steps:
- Make sure you know the force applied. This is usually given in newtons (N).
- Determine the distance the object moved while the force was applied, in meters (m).
- Calculate the work done (energy) in joules by multiplying the force by the distance: Work (J) = Force (N) × Distance (m).
- If the force is applied at an angle, use only the component of force in the direction of motion.
Summary:
Calculating joules using the work equation is straightforward and provides a clear way to understand how much energy is transferred when a force moves an object. It’s a basic calculation that is crucial to many areas of science and engineering. However, this method relies on a constant force being applied in a straight line, which doesn’t account for more complex movements or variable forces.
Electric Power and Time
Electric devices consume energy over time, and this consumption can be translated into joules using the power rating of the device and the duration of its use.
Detailed Steps:
- Identify the power rating of the device, typically given in watts (W).
- Record the time the device is in operation in seconds (s).
- Calculate the energy consumption in joules with the formula: Energy (J) = Power (W) × Time (s).
Summary:
This method is highly relevant for everyday life, as it can help consumers understand their energy usage and thereby manage their electricity costs. One limitation is the assumption that the device uses power at a constant rate, which isn’t always the case with all appliances.
Calorimetry in Chemistry
Calorimetry is a technique used in chemistry to measure the amount of heat absorbed or released during a chemical reaction, phase change, or mixing of substances, which can be expressed in joules.
Detailed Steps:
- Determine the specific heat capacity of the substance ?, usually in joules per gram per degree Celsius (J/g°C).
- Measure the mass of the substance (m) in grams (g).
- Measure the change in temperature (T) that occurs, in degrees Celsius (°C).
- Use the formula: Heat Energy (Q) = m × c × T to calculate the energy in joules.
Summary:
Calorimetry connects chemistry and energy, providing a direct way to measure the energy changes in reactions. It’s a more advanced application that can be very precise, but setting up and conducting such experiments requires careful control of variables and conditions.
Potential Energy
Potential energy relates to the position of an object within a field of force, such as gravity or electric fields.
Detailed Steps:
- For gravitational potential energy, determine the mass (m) of the object in kilograms (kg).
- Measure the height (h) from the ground in meters (m).
- Use the acceleration due to gravity (g), which is approximately 9.81 meters per second squared (m/s) on Earth.
- The formula for gravitational potential energy (U) is U = m × g × h. Calculate the result in joules (J).
Summary:
Understanding potential energy is critical for fields such as engineering and environmental sciences. The calculations are generally straightforward but assume that the value of ‘g’ is constant, which can vary depending on altitude and location on Earth.
Kinetic Energy
Kinetic energy accounts for the energy an object possesses due to its motion.
Detailed Steps:
- Measure the mass (m) of the object in kilograms (kg).
- Determine the velocity (v) of the object in meters per second (m/s).
- Use the formula for kinetic energy (KE): KE = 0.5 × m × v.
- Calculate the energy in joules (J).
Summary:
Kinetic energy calculations are essential in designing all types of transportation methods and for understanding the motion of objects. The primary downside is the need for precise measurements of mass and velocity, which can be challenging in real-world situations.
Food and Nutrition
The energy content of food is often given in calories, but it can also be expressed in joules to understand energy from a physics perspective.
Detailed Steps:
- Note the energy content of the food item in calories (cal).
- Use the conversion factor that 1 calorie equals approximately 4.184 joules.
- Multiply the calories by the conversion factor to determine the energy in joules (J).
Summary:
Translating nutritional information into energy units familiar to physics can provide a different perspective on what we consume. However, this simplified conversion may overlook the complex ways our bodies process and utilize food energy.
Physics of Collisions
In physics, understanding the energy during collisions can be quite informative, particularly in terms of determining the energy transferred or dissipated as heat, sound, or deformation.
Detailed Steps:
- Calculate the kinetic energy of each object before and after the collision using the mass and velocity.
- Use the conservation of energy principle to determine the total kinetic energy before and after the collision.
- The difference in energy will give you the energy transferred.
Summary:
This is a more advanced application that reveals the dynamics of collisions. This kind of calculation often requires understanding complex interactions and multiple objects, which can sometimes be simplified in theoretical scenarios.
Tips and Tricks for Accurate Measurements
- Always use consistent units when performing calculations to avoid errors.
- For devices that have variable power consumption, try to find out the average power used during the period you want to measure.
- In potential energy problems, consider factors that might affect ‘g,’ such as altitude or significant depth.
Frequently Asked Questions About Joules
-
What is the difference between a joule and a calorie?
A joule is the SI (International System of Units) unit for energy, while a calorie is a non-SI unit. The energy in one calorie equals approximately 4.184 joules. -
Can you convert kilowatt-hours to joules?
Yes, you can convert kilowatt-hours to joules. Since 1 watt is equal to 1 joule per second and there are 3,600 seconds in an hour, 1 kilowatt-hour equals 3.6 million joules (kWh × 3.6 × 10^6 = j). -
Why do we use joules instead of other units of energy?
The joule is the standard unit of energy in the International System of Units (SI). It is widely used because it provides a common standard and is based on fundamental physical quantities.
The joule is a versatile unit that helps us measure and understand the work done, heat transferred, and energy consumed in nearly all scientific and engineering contexts. It ties together ideas from physics, chemistry, and beyond, giving us the ability to quantify and compare the power of processes and objects in a uniform way. Whether you’re calculating the energy to lift a weight, the consumption of an electrical appliance, or the heat involved in a chemical reaction, understanding how to do so in joules brings clarity and precision to your understanding of energy.