Understanding the relationship between pH levels and the strength of acids and bases is vital in many scientific fields including chemistry, biology, and environmental science. The term “pKa” refers to the acid dissociation constant, a numerical value that helps scientists make predictions about chemical behavior. The lower the pKa, the stronger the acid. But what if you need to find the actual Ka, the acid dissociation constant, from the pKa value? Fret not; with simple mathematical steps, this can be achieved, paving the way for deeper insights into the nature of chemical solutions.
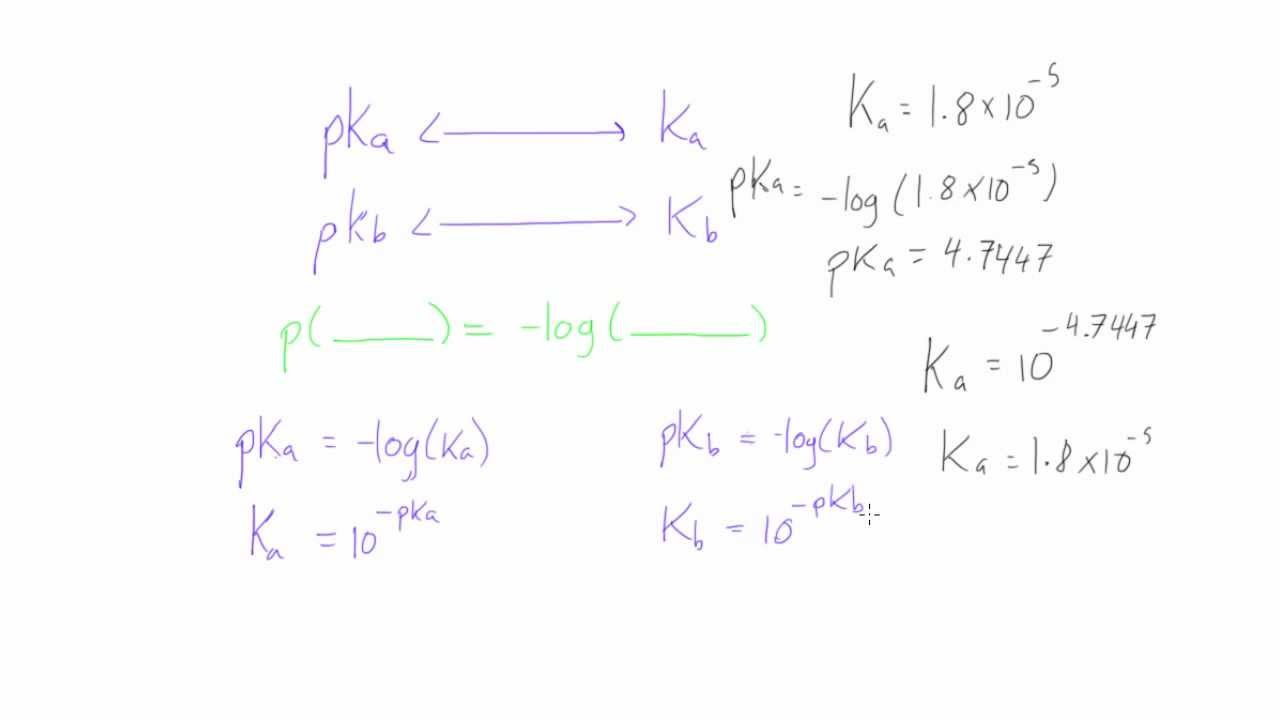
Conversion from pKa to Ka
A grasp of basic algebra is key to converting pKa to Ka. The pKa represents the negative logarithm (base 10) of the Ka value, which denotes a measure of how easily an acid releases a proton in solution.
Detailed Steps:
- Understand the basic relationship: pKa = -log(Ka). This equation is derived from the definition of pKa and the properties of logarithms.
- Rearrange the equation to solve for Ka: Ka = 10^(-pKa). This rearrangement is the algebraic method to “undo” a logarithm.
- Insert the pKa value: Replace the variable pKa in the formula with the pKa value you have.
- Perform the calculation: Use a calculator to raise 10 to the power of the negative pKa value. This gives you the Ka.
- Double-check your work: Ensure that your calculator is set correctly (for instance, in the right mode to handle scientific calculations).
Summary:
This method easily translates pKa to Ka, allowing you to assess an acid’s strength quickly. However, ensure accurate entry of the pKa value and set your calculator correctly to avoid errors.
Utilizing a pH Meter
Sometimes you are given the pH of a solution and the concentration of the acid and need to calculate the pKa first.
Detailed Steps:
- Obtain pH and concentration: Measure the pH of your solution using a pH meter and note the concentration of the acid.
- Calculate [H+]: Use the pH value to calculate the concentration of hydrogen ions by [H+] = 10^(-pH).
- Determine [A-]: If the concentration of the acid (HA) is given and you’ve found [H+], you can calculate [A-], the concentration of deprotonated acid, since [A-] = [H+] in a simple system.
- Use the Henderson-Hasselbalch equation: This equation is pKa = pH + log([HA]/[A-]) which can be rearranged to find pKa.
- Convert pKa to Ka: Now that you have pKa, use the first method to find Ka.
Summary:
While this approach also leads to finding Ka, it first requires the additional step of finding pKa using the pH and acid concentration, increasing the potential for error.
Through Titration Data
In a laboratory, titration provides the critical data to find the pKa through the midpoint of buffering.
Detailed Steps:
- Perform the titration: Titrate a known concentration of your acid with a strong base until it’s neutralized.
- Find the half-equivalence point: Review the titration curve to find the midpoint, which is where half of the acid is deprotonated.
- Note the pH at this point: The pH at the half-equivalence point is equal to the pKa.
- Translate pKa to Ka: Utilize the conversion from the first method to calculate Ka.
Summary:
Titration offers a practical approach to determine pKa, but it requires lab equipment and some familiarity with titration curves.
Estimate with Structure-Activity Relationship
The structure of an acid can give clues about its pKa, a concept used in medicinal chemistry.
Detailed Steps:
- Examine the acid’s structure: Look at the acid’s molecule for functional groups known to influence pKa.
- Compare with known values: Use literature to find pKa values of similar structures.
- Make an educated guess: Based on your comparisons, estimate the pKa.
- Proceed to find Ka: Use the conversion method described earlier to derive Ka.
Summary:
This method is more about estimation than precise calculation, and it depends heavily on the availability of comparative data.
Using Computational Software
Advanced software can predict pKa based on molecular structure.
Detailed Steps:
- Input the molecular structure: Use the software to draw or import the molecular structure of your acid.
- Run the pKa prediction tool: Follow the software instructions to estimate pKa.
- Convert to Ka: Apply the conversion method to find Ka from the predicted pKa.
Summary:
This modern approach is efficient and often accurate but requires access to specialized computational tools, which may not always be available.
General Tips and Tricks
Understanding acid-base concepts, maintaining proper lab techniques, or knowing how to work with related compute software will facilitate smooth calculations. Ensure your math skills are honed, double-check calculations, and always consider the context of your work for relevance and accuracy.
Reviewing Peer-Reviewed Journals
Often, you can find pKa values for common substances within scientific literature.
Detailed Steps:
- Search for your substance in journals: Look for published papers that mention your acid.
- Locate the pKa values: These are usually reported in the results or discussion sections.
- Convert to Ka: Use the pKa to Ka conversion technique.
Summary:
This method relies on the availability and accuracy of published data and might not be applicable for new or less-studied substances.
Seek Expertise
In some cases, consulting with a chemist or professor could be the fastest way to find or confirm Ka values.
Detailed Steps:
- Identify a reference: Locate someone knowledgeable in chemistry.
- Present your data: Share the information you have on the acid.
- Discuss the pKa and Ka: Use their expertise to validate your values or provide a more accurate pKa.
- Calculate Ka as before: With professional input, you can be more confident in your translations from pKa to Ka.
Summary:
While this method benefits from expert validation, it isn’t a direct calculation method and relies on the availability and willingness of experts to assist.
Practical Applications
Understanding how to connect pKa and Ka is not just academic; it’s directly applicable in product development, environmental analysis, pharmacology, and many other fields.
Detailed Steps:
- Associate pKa/Ka with your field: Gather context on how these values are used in your specific industry.
- Apply the calculation: Use the relevant method to find your Ka value.
- Utilize the data: Interpret the implications of the Ka in your practical application.
Summary:
This comprehensive approach integrates the Ka calculation into usable, industry-specific knowledge, but it requires higher-level understanding of both chemistry and the business or scientific field in question.
Routine Practice
The key to mastering any calculation is practice.
Detailed Steps:
- Find practice problems: Use textbooks or online resources.
- Work through them: Apply the methods outlined above regularly.
- Check your answers: Confirm your calculations with answer keys or software.
Summary:
Consistent practice reinforces accuracy but may require a time commitment and access to quality practice materials.
Conclusion
Calculating Ka from pKa may initially seem daunting, but with these methods and tips, the task becomes more accessible. By understanding the underpinnings of acid-base chemistry and applying straightforward steps, anyone can translate pKa to Ka, a process integral in numerous scientific inquiries and applications.
FAQs:
-
What is pKa?
- pKa is the negative logarithm of the acid dissociation constant (Ka) and provides a measure of the strength of an acid.
-
Why is it important to convert pKa to Ka?
- Converting pKa to Ka aids in determining an acid’s dissociation strength in a more direct manner, which is essential for scientific experiments and formulations.
-
Can I find Ka values without having pKa?
- It’s possible by using other methods like titration, but having the pKa simplifies and speeds up the process.
-
How accurate are computational pKa predictions?
- Computational predictions can be quite precise but are dependent on the algorithm and data used by the software.
-
Is it possible to estimate pKa from molecular structure?
- Yes, through an understanding of structural attributes that influence acidity, one can estimate pKa, but this requires substantial expertise in chemistry.