In the fascinating world of chemistry, understanding the strength of acids and bases is paramount. It often boils down to two critical values: the acid dissociation constant (Ka) and the base dissociation constant (Kb). These constants are like secret codes, revealing how much an acid or base can donate or accept protons in a water solution. Calculating Kb from Ka is a helpful tool for scientists and students alike, as it enables them to predict chemical behavior in a reaction. To make it simple, think of it as converting a set of instructions in a manual from one language to another.
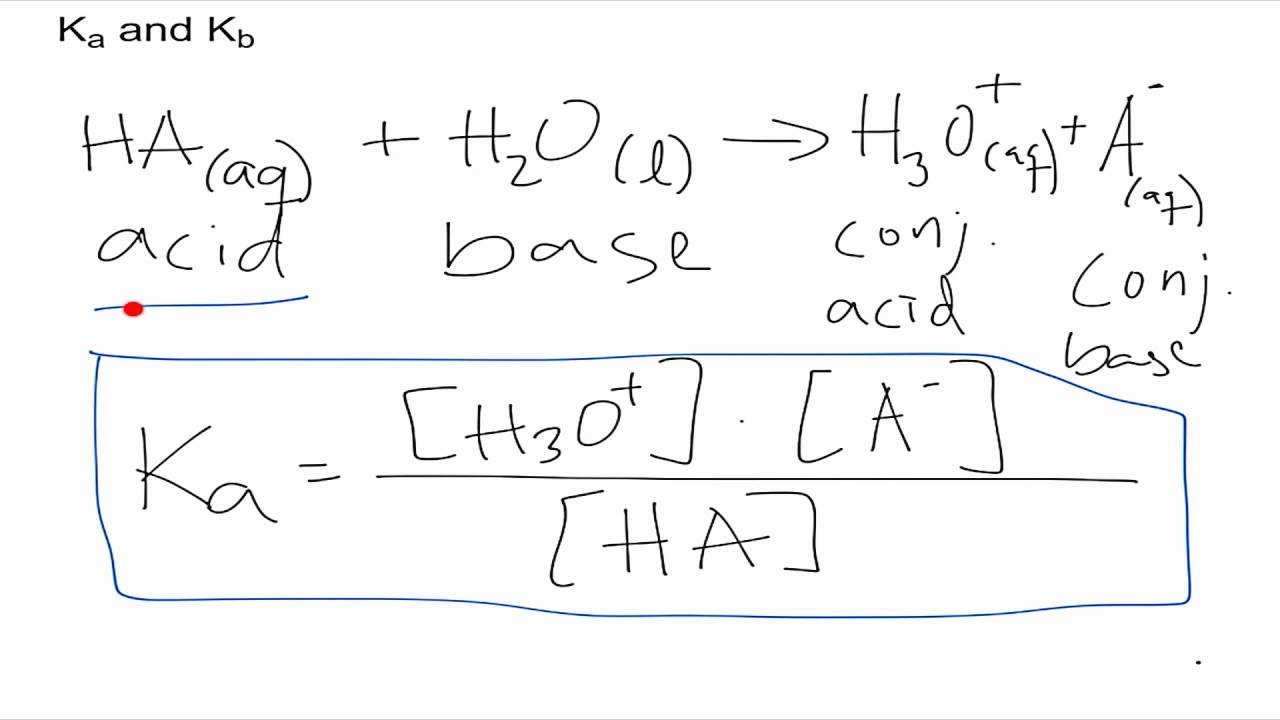
Using the Relationship Between Ka and Kw
To start off, knowing the relationship between Ka and Kb is crucial because it’s rooted in a very important concept in chemistry known as the ion product constant for water (Kw). Kw is a constant at a given temperature, which is 1 x 10^-14 at 25°C.
Detailed Introduction
In a water solution at 25°C, the product of Ka and Kb for a conjugate acid-base pair equals Kw. This provides a neat mathematical connection between the acid and base strengths of a pair.
Detailed Steps
- Write down the known Ka value of the acid.
- Remember that Kw is 1 x 10^-14 at 25°C. Note this down.
- Use the following formula: Kb = Kw / Ka.
- Divide Kw by the Ka value you have.
- Now, you have the Kb value for the conjugate base of the acid.
Summary
This straightforward method allows you to quickly find the Kb value for a conjugate base if you know the Ka of its acid. This calculation is fundamental in predicting the behavior of the base in reactions. However, it is temperature dependent, so adjustments need to be made if you’re working at temperatures other than 25°C.
Utilizing pKa and pKb Values
Understanding pKa and pKb is key as they are the logarithmic scales of Ka and Kb. They help in simplifying the calculations when dealing with very small or large numbers.
Detailed Introduction
pKa and pKb give us a more manageable set of numbers to work with, circumventing the cumbersome use of scientific notation in many instances.
Detailed Steps
- Start with the known pKa value of the acid.
- Recall that pKw, like Kw, is always 14 at 25°C.
- Use the relationship: pKb = pKw – pKa.
- Subtract the pKa value from pKw.
- The result is the pKb value for the conjugate base.
Summary
Converting pKa to pKb using this method simplifies the process and is especially useful when dealing with very strong or weak acids and bases. Yet, this approach still relies on temperature constancy (25°C) and requires an understanding of logarithmic functions, which can be an additional learning curve for some.
The Role of Temperature in Ka and Kb
Temperature plays a significant role in the value of Kw, and consequently affects the relationship between Ka and Kb.
Detailed Introduction
Reactions are temperature-dependent, and as Kw changes with temperature, so does the balance between Ka and Kb. It’s important to understand how these changes can impact chemical equilibria in different conditions.
Detailed Steps
- Determine the temperature at which your reaction occurs, if it’s not 25°C.
- Find the value of Kw at this new temperature from a chemical handbook or a reliable resource.
- Note down the Ka of the acid at the new temperature, if available.
- Calculate Kb by dividing the new Kw by the Ka value (Kb = Kw / Ka).
Summary
Adjusting calculations for temperature is vital for accurate predictions in real-world scenarios, but this requires access to specialized data and a deeper understanding of temperature effects on chemical equilibria.
Considering the Strength of the Acid or Base
The stronger the acid, the weaker its conjugate base, and vice versa. This concept helps in understanding and predicting the relative strength of a conjugate base through its acid’s Ka value.
Detailed Introduction
This principle is a reflection of the balance within an acid-base conjugate pair. When an acid donates a proton easily (indicating strength), its conjugate base will be less likely to accept a proton back, revealing its comparative weakness.
Detailed Steps
- Gauge the strength of your acid by its Ka value; a larger Ka means a stronger acid.
- Understand that a stronger acid will have a weaker conjugate base.
- Calculate the conjugate base’s relative Kb value using the Kw / Ka relationship, if a specific value is needed.
Summary
Comprehending the inverse relationship between an acid’s strength and its conjugate base’s strength is valuable for theoretical predictions and understanding chemical reactions. However, this does not provide a specific Kb value unless calculations are performed.
Analysis of Conjugate Acid-Base Pairs
Analyzing conjugate acid-base pairs is essential to predict how a substance will behave under various acidic or basic conditions.
Detailed Introduction
Every acid has a conjugate base, and every base has a conjugate acid. By understanding these pairs, you can often estimate how a substance will act in a solution without doing any calculations.
Detailed Steps
- Identify the conjugate base of the acid in question.
- Use known information about the pair to assess the likely behavior of the base.
- If needed, calculate the exact Kb value using formulas provided earlier.
Summary
Understanding conjugate pairs allows for educated guesses in chemical behavior and reactions. This approach offers a solid theoretical framework, but for experimental accuracy, actual calculations are necessary.
Acid-Base Neutralization Reactions
Acid-base neutralization reactions can also provide insights into the relative strengths of acids and bases, often simplifying the conceptual understanding of Ka and Kb.
Detailed Introduction
In a neutralization reaction, an acid and a base react to form water and a salt. The reaction can indicate which substances are strong or weak acids or bases, based on how completely they react.
Detailed Steps
- Observe an acid-base neutralization reaction.
- Note which acid and base react completely to form water and a salt.
- Infer that a strong acid or base will completely dissociate, indicating a higher Ka or Kb value.
Summary
Neutralization reactions give a practical understanding of acid and base strengths. While this observational method offers a good starting point, it won’t provide precise Ka or Kb values without additional data.
Computational Chemistry Software
Advancements in computational chemistry allow for the use of software to predict and calculate Ka and Kb values with high accuracy.
Detailed Introduction
Specialized software can simulate chemical reactions and calculate the equilibrium constants for acids and bases, taking into account various factors that may be difficult to manually calculate.
Detailed Steps
- Find a reliable computational chemistry tool or software.
- Input the molecular structure of the acid or base in question.
- Run simulations to predict the Ka or Kb values for the substance.
Summary
Using computational tools can provide high precision in calculations and save a significant amount of time. However, these tools can be complex and expensive, and they require a certain level of expertise to use effectively.
pH Measurements and the Henderson-Hasselbalch Equation
The pH of a solution can be used alongside the Henderson-Hasselbalch equation to find Ka or Kb values by measuring the degree of dissociation of the acid or base.
Detailed Introduction
This relationship offers a more experimental approach in determining the strength of an acid or base. It is an especially good option if computational tools are not available, or if an empirical approach is preferred.
Detailed Steps
- Measure the pH of a solution containing the acid or base whose Ka or Kb you want to find.
- Use the Henderson-Hasselbalch equation: pH = pKa + log([A-]/[HA]), where [A-] is the concentration of the conjugate base and [HA] the concentration of the acid.
- Rearrange the equation to solve for pKa (and consequently Ka) or pKb (and consequently Kb) as needed.
Summary
This method provides a quantitative connection between pH and Ka or Kb values and can be very accurate, but it requires precise measurements and controlled conditions.
Laboratory Titration
Titration is a laboratory technique that can help determine the Ka or Kb by precisely measuring how much acid or base is required to neutralize a solution.
Detailed Introduction
During a titration, a substance with a known concentration (titrant) is slowly added to a substance with an unknown concentration (analyte), until the reaction is complete. The point of neutralization provides key insight into the strength of the analyte.
Detailed Steps
- Set up a titration with your acid or base and a titrant of known concentration.
- Carefully measure the volume of titrant used to reach the neutralization endpoint.
- Use the titration curve and calculations to determine the Ka or Kb value of the analyte.
Summary
Titration is a hands-on and accurate method for finding Ka or Kb, beneficial for educational purposes and in-person demonstrations. The technique is fairly accessible, though it requires a well-equipped laboratory and a certain level of chemical knowledge to interpret the results.
Consulting Academic and Chemical Databases
For many common substances, Ka and Kb values have already been extensively studied and are available in academic and chemical databases.
Detailed Introduction
Instead of calculating values from scratch, leveraging the vast amount of data already available can be a quick and reliable alternative, especially for widely-used or well-researched chemicals.
Detailed Steps
- Access an academic or chemical database with entries for the substance of interest.
- Search for the Ka or Kb value directly, or find related equilibrium constant information.
- Use these values in your calculations or analysis as needed.
Summary
Using databases is efficient and practical for quickly finding accurate data. However, this method relies on the availability and accuracy of the database information, which might not always be up-to-date or include every substance.
Academic Collaboration
Collaborating with academic institutions or professionals in the field of chemistry can provide valuable insights and resources for calculating Ka and Kb values.
Detailed Introduction
The exchange of knowledge and experience between non-experts and experts in academia can facilitate better understanding and more accurate results for complex chemical calculations.
Detailed Steps
- Reach out to university departments, professors, or researchers in chemistry.
- Discuss your requirements and collaborate on the Ka or Kb calculations.
- Utilize the expertise and resources available within the academic setting to calculate your values.
Summary
Academic collaboration opens doors to expert knowledge and sophisticated equipment, possibly yielding the most reliable results. This method, while advantageous, can be time-consuming and dependent on the willingness of academic partners to collaborate.
In conclusion, navigating the world of acid and base strengths through Ka and Kb calculations is a delicate balancing act that draws upon a deep understanding of chemical concepts and a variety of calculation methods. There is no one-size-fits-all approach; different situations may require different methods, each with their own benefits and limitations. The beauty of chemistry lies in its complex yet interconnected nature, where foundational principles pave the way for discovery and understanding. From leveraging computational tools to hands-on laboratory titrations, the journey of exploration in this field is both rich and rewarding.
FAQs:
-
What is the significance of calculating Kb from Ka?
Calculating Kb from Ka lets us predict how a base will behave in solution based on its conjugate acid’s properties, which is invaluable in chemical synthesis, research, and education. -
Why do we use pKa and pKb instead of Ka and Kb sometimes?
pKa and pKb offer a more manageable set of numbers to work with by converting very small or large equilibrium constants to a logarithmic scale, simplifying calculations and comparisons. -
Is the calculation of Kb from Ka temperature-dependent?
Yes, the relationship between Ka and Kb is dependent on the temperature because it involves the ion product constant of water (Kw), which varies with temperature.







