Understanding the molar solubility of a substance is like figuring out how much sugar you can dissolve in your coffee before it can’t take any more and leaves the excess at the bottom. It’s a basic yet crucial concept in chemistry that tells you how much of a substance can be dissolved in a solvent until no more can be added. This knowledge is particularly useful in applications ranging from pharmaceuticals to environmental science. The calculations can seem complex, but with a step-by-step approach, anyone can grasp the fundamentals of molar solubility.
Understanding Equilibrium Constants
Before we dive into calculating molar solubility, it’s essential to understand equilibrium constants. These constants tell us how likely a compound is to dissociate—break apart into ions—when dissolved in water.
Detailed Steps:
-
Identify the chemical equation of the dissolution process. For example, ( CaF_2 (s) leftrightarrow Ca^{2+} (aq) + 2F^{-} (aq) ).
-
Write down the expression for the equilibrium constant, known as the solubility product constant ( (K_{sp}) ). For ( CaF_2 ), this would look like:
[ K_{sp} = [Ca{2+}][F{-}]^2 ] -
Find the value of ( K_{sp} ) from a reference table, which has been experimentally determined for many compounds.
Summary:
Understanding equilibrium constants is an excellent first step because it sets the foundation for molar solubility calculations. However, it might get confusing when dealing with more complex salts and their ions.
Basic Ion Dissociation
Knowing how to approach simple ion dissociation is the next logical step. This can be used where salts break down into two types of ions.
Detailed Steps:
-
Set up an expression for the dissolution, spelling out how the salt dissociates into its ions. For ( AgCl ), it would be: ( AgCl (s) leftrightarrow Ag^+ (aq) + Cl^- (aq) ).
-
Create an ICE table (Initial, Change, Equilibrium) to keep track of ion concentration changes during dissolution.
-
Apply the ( K_{sp} ) constant to find the concentrations of each ion at equilibrium. This involves algebraic manipulation, typically assuming that initial concentrations of ions are zero.
Summary:
This method simplifies the process for salts that produce one type of cation and one type of anion. It becomes slightly more complex when dealing with substances that produce either more than one type of ion or different amounts of cations and anions.
Complex Ion Formation
Sometimes salts interact with their solvent to form more complex ions. Understanding these reactions is crucial to accurately find molar solubilities for such compounds.
Detailed Steps:
-
Write the balanced equation for the reaction including the complex ion formation. For example, silver chloride in ammonia solution forms ( [Ag(NH_3)_2]^+ ).
-
Use the equilibrium constants for the formation of complex ions, known as formation constants (( K_f )), to determine the concentration of complex ions in solution.
-
Combine the necessary equilibrium expressions to solve for the molar solubility, accounting for the complex ion.
Summary:
The inclusion of complex ions introduces an additional layer of complexity to our calculations. While this method expands our capability to calculate molar solubility for a wider range of substances, it requires a stronger grasp of chemical equilibria and algebra.
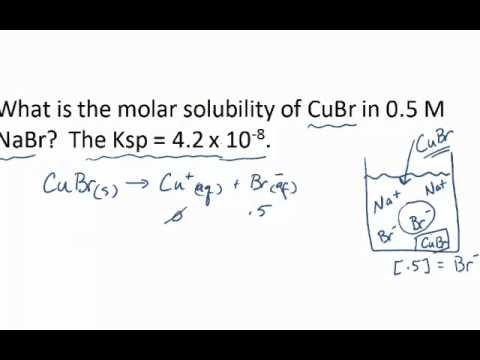
Common Ion Effect
The presence of a common ion can significantly impact the molar solubility of a compound—a concept known as the common ion effect.
Detailed Steps:
-
Recognize if a common ion is present in your solution, either from the solvent or added from another source.
-
Adjust your equilibrium calculations to consider the added concentration of the common ion that can shift the dissolution equilibrium.
-
Solve for the new molar solubility of your compound using the modified equilibrium expression.
Summary:
The common ion effect is handy for predicting how the solubility of compounds changes in different environments. However, it adds a layer of complexity as you now have to account for additional ions in your calculations.
Impact of pH on Solubility
The acidity or basicity of a solution, its pH, can also affect the solubility of a substance, particularly when the substance can react with hydronium or hydroxide ions.
Detailed Steps:
-
Consider if the ion can react with ( H^+ ) or ( OH^- ) ions—such as carbonate reacting with ( H^+ ) to form ( HCO_3^- ).
-
Set up simultaneous equilibrium expressions for the solubility and the acid/base reaction.
-
Solve the combined set of equations to find the molar solubility under the given pH conditions.
Summary:
The impact of pH on solubility highlights the interaction between chemical equilibria and acidity/basicity of the solution, which can be complex but offers a nuanced understanding of solubility in various conditions.
Temperature’s Role
Temperature can also affect solubility, though calculating these changes often requires additional thermodynamic data.
Detailed Steps:
-
Understand how temperature affects solubility: Generally, the solubility of gases in liquids decreases with increasing temperature, while the solubility of solids in liquids often increases.
-
Refer to solubility tables or conduct experiments to obtain data on the effect of temperature change on your substance.
-
Adjust calculations accordingly based on the observed changes in solubility related to temperature.
Summary:
This approach introduces empirical data that might not be readily available for all substances. Yet, it’s crucial for processes sensitive to temperature changes, such as in industrial and environmental applications.
Molar Solubility from Ksp Values
Direct calculation of molar solubility from known ( K_{sp} ) values is the most straightforward method.
Detailed Steps:
-
Use the ( K_{sp} ) value for your salt. As an example, ( CaF_2 ) with ( K_{sp} = 3.9 times 10^{-11} ).
-
Assume molar solubility (s) for your substance, where the equilibrium concentration of ( Ca^{2+} ) is ( s ) and ( F^- ) is ( 2s ).
-
Solve for ( s ) using the expression ( K_{sp} = s(2s)^2 ) and algebraic manipulation.
Summary:
Calculating molar solubility directly from ( K_{sp} ) values is ideal for straightforward compounds and is often the first technique taught to beginners.
Saturation Levels and Precipitation
Understanding when a solution will begin to precipitate—when the dissolved substance starts to form a solid—is a practical application of solubility.
Detailed Steps:
-
Calculate the ion product—the actual concentrations of ions in solution—and compare it to the ( K_{sp} ).
-
Determine if precipitation occurs by seeing if the ion product exceeds ( K_{sp} ), indicating a supersaturated solution.
-
Adjust the solution conditions to prevent or promote precipitation as necessary for your application.
Summary:
This technique is central to many industrial processes and environmental assessments, though it requires precise concentration measurements and sometimes complex calculations.
Stoichiometry of Dissolution
The stoichiometry—or the proportion of elements involved in a reaction—can impact the molar solubility, especially in more complex ions.
Detailed Steps:
-
Start with the balanced equation for the dissolution process, paying close attention to the stoichiometry of the reactants and products.
-
Create an ICE table to account for the stoichiometric coefficients of each ion in the expression for ( K_{sp} ).
-
Solve for the molar solubility while considering the stoichiometric ratios.
Summary:
This method accentuates the need to consider the ratios of ions produced in dissolution, which is vital for understanding the solubility of various compounds, though it might require a deeper understanding of chemical reactions.
Now that we have walked through various ways to calculate and understand molar solubility let’s consolidate our learning. Molar solubility is an expression of concentration and is crucial for many practical applications like medicine formulation and environmental conservation. Grasping this concept allows one to predict the behavior of substances in various conditions, facilitating better control over their use and management. While the calculations can at first seem daunting, they become much more approachable when broken down into logical steps. As with any skill, practice and patience are key to mastering molar solubility calculations.
In conclusion, we’ve seen that while the principles underlying molar solubility calculations are rigorous, the steps to find these values can be methodical and accessible. By taking the time to understand the components of solubility, even those without a strong technical background can grasp how to quantify the solubility of compounds, leading to practical insights in a range of fields.
FAQs:
1. What is molar solubility?
Molar solubility is the number of moles of a solute that can be dissolved per liter of solution before the solution becomes saturated.
2. How does temperature affect solubility?
Temperature can increase the solubility of most solids in liquids but typically decreases the solubility of gases in liquids. The exact effect will depend on the substance in question.
3. What is the common ion effect and how does it affect solubility?
The common ion effect occurs when a compound is dissolved in a solution that already contains one of the ions that the compound dissociates into. This can decrease the compound’s solubility due to a shift in the equilibrium towards the undissociated compound.









