Introduction:
Understanding the amount of a substance in a chemical reaction is crucial for laboratory work, industry processes, and even cooking recipes. You might have come across the term “mole” in science. It’s a basic unit in chemistry that helps quantify the amount of a substance. A mole can be described as a collective bunch just like a dozen, except it’s a much larger number—exactly 6.022 x 10^23 particles! When you’re baking and need three eggs, think of needing three moles of a substance for a reaction. This guide will simplify the process of calculating moles, ensuring that you’ll be able to do it with ease and confidence.
Using the Molar Mass
When converting grams of a substance to moles, the molar mass is your best friend. It is the weight of one mole of a substance, typically expressed in grams per mole (g/mol), and can be found on the periodic table.
- Identify the chemical formula of the substance.
- Look up the atomic mass of each element in the chemical formula from the periodic table.
- Multiply the atomic mass of each element by the number of times it appears in the formula.
- Add up all the masses to get the molar mass.
- Measure the mass of your substance in grams.
- Divide the mass of the substance by the molar mass to calculate moles.
Summary:
This method is fundamental and accurate, making it a staple in any chemical calculations. However, it can be tricky if one is not familiar with using the periodic table or if the substance is a complex molecule with many different elements.
Counting Particles
If you have a known number of individual particles (atoms, molecules, ions, etc.), you can easily convert that number to moles.
- Count or obtain the number of particles of the substance.
- Use Avogadro’s number (6.022 x 10^23) as a conversion factor.
- Divide the number of particles by Avogadro’s number to get the number of moles.
Summary:
This process is straightforward when the number of particles is given, but counting particles one by one is not practical or possible for large numbers, making this technique limited to theoretical problems or those involving Avogadro’s number.
Through Concentration and Volume
Solutions in chemistry often have a concentration given in moles per liter (M, mol/L). When you have a solution of known concentration, the volume can guide you to the moles contained in that volume.
- Record the concentration of the solution (Molarity, M).
- Measure the volume of the solution in liters (L).
- Multiply the concentration by the volume to find the number of moles.
Summary:
This is a highly practical method for liquid solutions and ease-for-use is a major benefit. However, accurately measuring liquid volumes requires care, and the concentration must be accurately known for the calculation to be precise.
Using Gas Laws
For gaseous substances, the Ideal Gas Law is a handy equation that relates the volume, temperature, pressure, and amount of gas.
- Identify the volume, pressure, and temperature of the gas.
- Use the Ideal Gas Law equation: PV = nRT, where P is pressure, V is volume, n is moles, R is the ideal gas constant, and T is temperature in Kelvin.
- Solve the equation for n (moles) based on your known variables.
Summary:
The benefits of this method are that it links physical properties to the amount of substance, invaluable for gas reactions. Drawing back, it requires an understanding of gas behavior and the correct use of units.
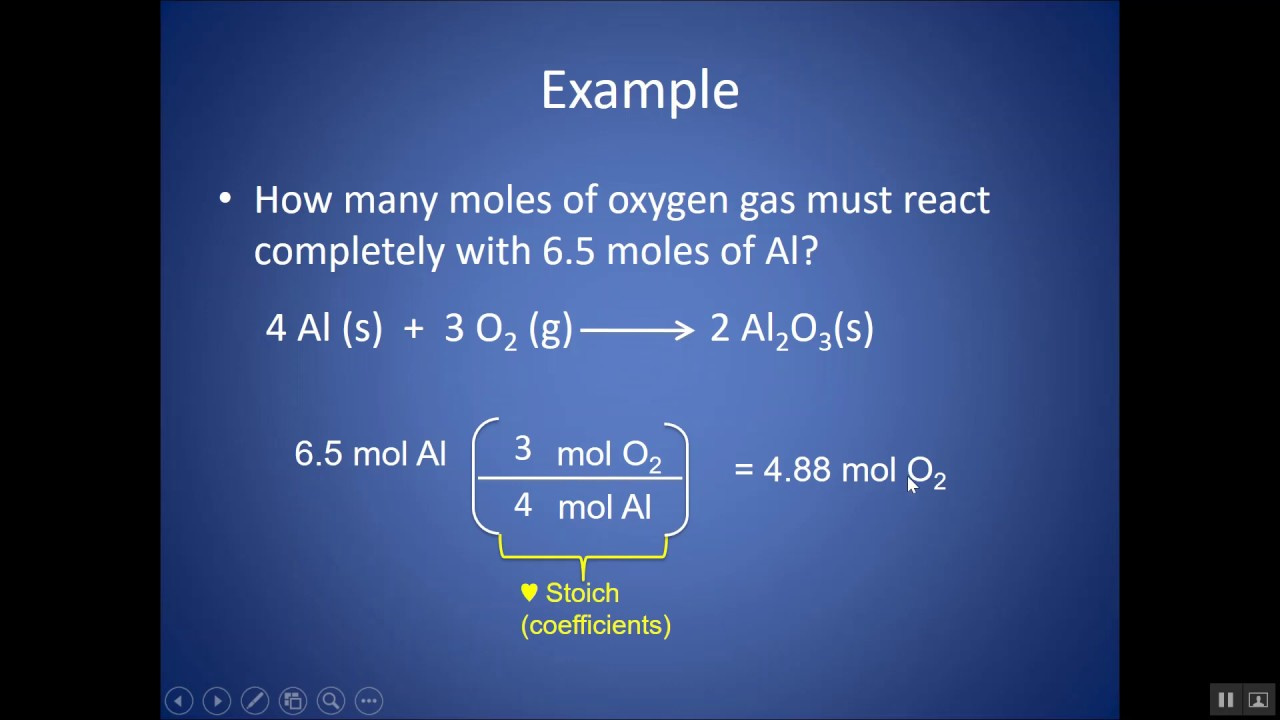
Estimating from a Balanced Equation
If you’re working with chemical reactions, stoichiometry and the balanced equation for the reaction can tell you how many moles of each substance are involved.
- Write the balanced equation for the chemical reaction.
- Determine the molar ratios from the balanced equation.
- Calculate the moles of one substance.
- Use the molar ratios to find the moles of other substances.
Summary:
Stoichiometry is powerful for predicting the outcomes of reactions and for planning experiments. However, this requires a thorough understanding of chemical reactions and balance.
Through Titration
Titration is a laboratory method for finding the concentration of a solution by reacting it with a solution of known concentration.
- Prepare the solution of unknown concentration and the titrant (solution of known concentration).
- Measure an initial volume of titrant.
- Add the titrant to the unknown solution until the reaction is complete (indicated by a color change or pH change).
- Measure the final volume of titrant.
- Calculate the change in volume and use stoichiometry to find the moles of the unknown substance.
Summary:
This method is precise and allows for determining the concentration of a substance in a mixture. The downside is that it requires specific equipment and indicators, as well as a proper understanding of stoichiometry.
Electrolysis
In electrolysis, an electric current is passed through a substance causing a chemical change. The amount of substance produced or consumed can be related to the amount of electric charge passed.
- Measure the current (I, in amperes) and the time (t, in seconds) during which current is passed.
- Use the formula Q = It, where Q is the charge in coulombs.
- Apply Faraday’s laws of electrolysis to relate the charge to the moles of substance.
Summary:
Useful for processes involving electric current, it demands understanding Faraday’s laws and involves equipment to measure electric current and time.
Reactant in Excess
When one reactant is in excess in a chemical reaction, it means that there will be some of the reactant left over after the reaction is complete. You can use the limited reactant to find the number of moles of the product.
- Determine which reactant is in excess through stoichiometry.
- Calculate the moles of the limiting reactant.
- Use the balanced equation to find the moles of substance produced.
Summary:
Determining reactant in excess is crucial for maximizing yield in industrial processes, though deciphering the limiting reactant and performing stoichiometry can be complex for beginners.
Using pH
The pH of a solution can provide information on the concentration of hydrogen ions, which can be converted to moles.
- Measure the pH of the solution.
- Use the pH to find the hydrogen ion concentration (pH = -log[H+]).
- Calculate the moles of hydrogen ions using the volume of the solution and its hydrogen ion concentration.
Summary:
This method is particularly useful for acid-base reactions, but requires precision pH measurement and understanding of logarithmic functions.
Limiting Reagent Principle
When performing a chemical reaction with multiple reactants, the limiting reagent principle helps determine the amount of product that will form.
- Calculate the moles of all reactants.
- Use stoichiometry to find out which reactant will be consumed first—it’s the limiting reagent.
- The moles of the limiting reagent will determine the maximum moles of product that can form.
Summary:
This principle is essential for stoichiometric calculations, though it presupposes a good grasp of chemical equations and stoichiometry.
Conclusion:
Calculating moles is a fundamental skill for understanding and performing chemical reactions. This guide provides a curated list of methods to measure the number of moles, accounting for various states of matter and reaction conditions. Whether dealing with solids, liquids, gases or solutions, these approaches offer versatility and precision. However, a common thread is the necessity to understand the underlying principles, such as stoichiometry, the behavior of gases, and basic chemical reactions. Mastering these concepts will make the calculation of moles a much more intuitive process.
FAQs:
Q1: What is a mole in simple terms?
A1: A mole is a unit in chemistry that represents a very large number of particles—6.022 x 10^23 of them. It’s like a dozen, but for atoms, molecules, or other particles.
Q2: Why do chemists use moles instead of counting individual particles?
A2: Chemists use moles because counting individual particles is impractical due to their tiny size and the huge numbers involved in chemical reactions. Moles provide a manageable way to express quantities.
Q3: Can the molar mass be determined for compounds as well as for elements?
A3: Yes, the molar mass of a compound is calculated by adding the molar masses of the individual elements that make up the compound, adjusted for the number of atoms of each element in the compound formula.







