In the vast and exciting world of chemistry, one fundamental skill that stands out is the ability to calculate theoretical yield. This concept is at the heart of practical chemistry—whether you’re a student running your first reaction in the lab or a professional chemist optimizing a manufacturing process. The theoretical yield is the maximum amount of product that can be generated from a given amount of reactants, according to stoichiometric principles and assuming perfect conditions. Grasping this concept not only sharpens your understanding of chemical reactions but also allows for efficient resource planning and management within any chemical work.
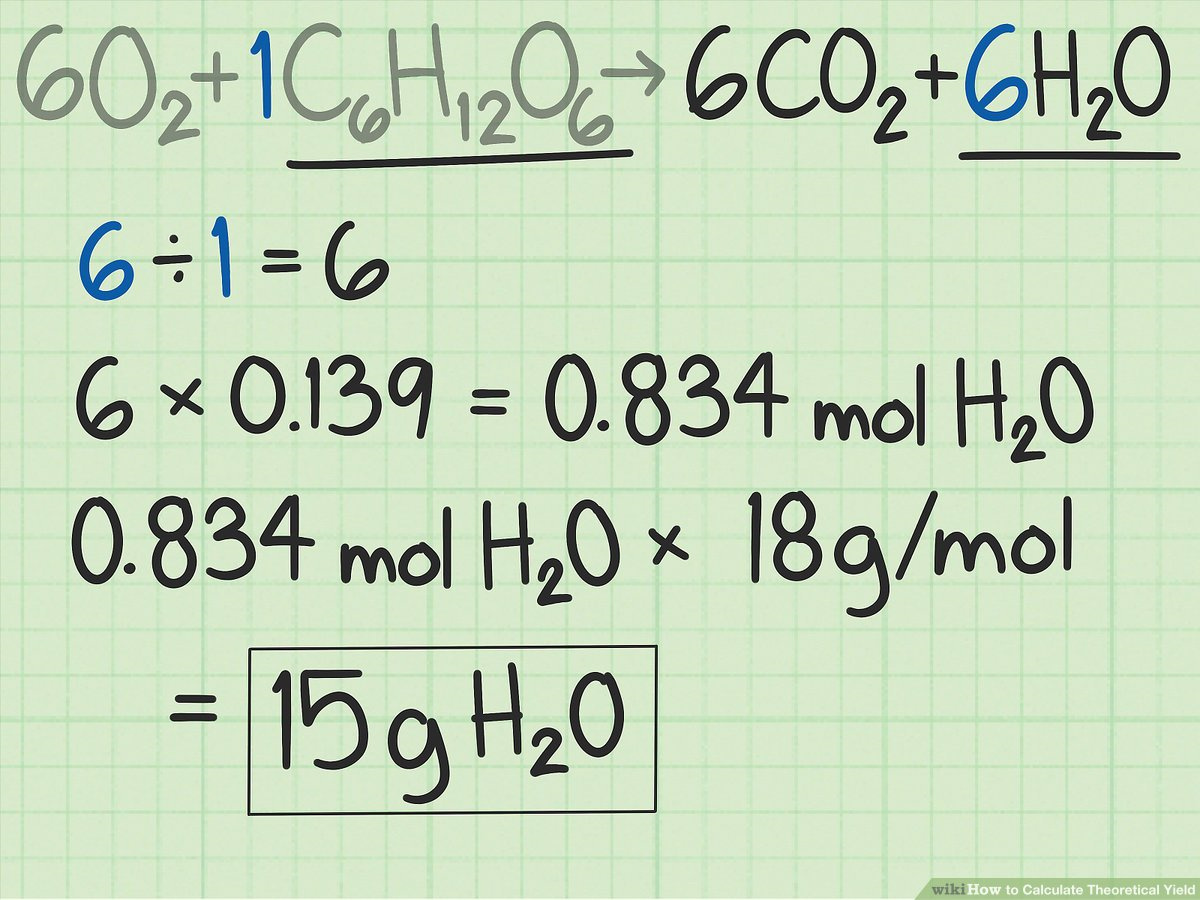
Understanding Stoichiometry
In the realm of chemistry, stoichiometry is the mathematical relationship between the amounts of reactants and products in a chemical reaction. It is based on the balanced chemical equation, which tells us the ratio in which substances react and form products. Knowing this relationship helps in predicting the amounts of products formed from given quantities of reactants.
Detailed Steps:
- Identify the Balanced Equation: Find the chemical equation for the reaction and ensure it is balanced. The number of atoms for each element must be the same on both sides of the equation.
- Determine the Molar Masses: Calculate the molar mass of each reactant and product by adding the atomic masses of all atoms in a molecule.
- Convert Mass to Moles: Use the molar mass to convert the grams of reactants to moles, as stoichiometry is based on mole ratios.
- Use the Mole Ratio: Apply the mole ratio from the balanced equation to find the number of moles of product that could be formed from the reactants.
- Convert Moles to Grams: Use the molar mass of the product to convert the theoretical number of moles back to grams. This gives you the theoretical yield.
Summary:
Understanding stoichiometry is crucial for accurately calculating the theoretical yield. It ensures precise conversions between mass and moles, aligning with the mole ratio. The main benefit is that it provides an exact framework for estimating expected outcomes; however, it assumes ideal conditions that might not always mirror practical scenarios.
Limiting Reactant Identification
In any chemical reaction with two or more reactants, one reactant will run out first, limiting the amount of product that can be formed. This reactant is known as the limiting reactant, and identifying it is essential for calculating the theoretical yield.
Detailed Steps:
- Calculate the Moles of Each Reactant: Using the molar mass, convert the mass of each reactant to moles.
- Divide by the Stoichiometric Coefficients: For each reactant, divide the number of moles by its coefficient in the balanced equation.
- Identify the Limiting Reactant: The reactant with the smallest resulting value from step 2 is the limiting reactant.
Summary:
Determining the limiting reactant is invaluable as it directly impacts the theoretical yield, only permitting calculations based on this specific reactant. However, difficulties may arise in accurately measuring reactant quantities, which might lead to errors in identifying the limiting reactant.
Applying Percentage Purity
Impurities in reactants can affect the amount of product formed. Thus, considering the purity of reactants is an essential step toward an accurate calculation of the theoretical yield.
Detailed Steps:
- Determine the Purity: Check the purity percentage given for the reactants.
- Adjust the Reactant Quantity: Multiply the total mass of each reactant by its purity percentage to find the effective mass of the reactant.
- Recalculate Moles: Convert this effective mass to moles for use in the theoretical yield calculation.
Summary:
Adjusting for percentage purity refines the theoretical yield calculation, accounting for non-reactive components within reactants. However, precisely measuring purity levels can be challenging and may introduce some uncertainty into the yield calculation.
Excess Reactant Calculation
Not all reactants are used up in a chemical reaction; some may be present in excess. Understanding the role of excess reactants helps to better comprehend their role in yield calculations.
Detailed Steps:
- Identify the Excess Reactant: After finding the limiting reactant, the others are considered to be in excess.
- Calculate the Theoretical Yield Based on the Limiting Reactant: Only consider the moles of the limiting reactant when using the stoichiometric ratio to calculate the yield.
Summary:
Recognizing excess reactants solidifies the understanding that only the limiting reactant’s amount governs the theoretical yield. The primary benefit is avoiding unnecessary calculations with non-limiting reactants, but this approach assumes all excess reactants do not affect the reaction, which isn’t always the case.
Effects of Temperature and Pressure
Temperature and pressure conditions can influence the outcome of chemical reactions and, consequently, the theoretical yield. It’s important to understand the general effects these conditions could have on a reaction.
Summary:
Grasping the roles of temperature and pressure is more about recognizing potential variances than direct calculations. These factors could alter reaction rates and favor different products, but this consideration is mainly relevant for reactions sensitive to changes in conditions and is not typically a major concern when calculating theoretical yields.
Use of a Yield Calculator
For those less inclined towards manual calculations, yield calculators are available online to simplify the process. These tools require data input to automatically generate a theoretical yield.
Summary:
Yield calculators streamline the calculation process, offering a quick, user-friendly alternative to manual computations. They are particularly useful for those uncomfortable with the math involved. However, they limit the user’s deeper understanding of the underlying chemistry concepts.
Review with Practice Problems
Practice reinforces learning, and working through example problems can build confidence in calculating theoretical yield.
Summary:
Practice problems are a great way to solidify understanding and identify any areas of confusion. The more one practices, the easier it will be to overcome calculation challenges, although practice scenarios may not encompass all complexities of a real-world application.
In conclusion, calculating theoretical yield is a fundamental aspect of chemistry that bridges theoretical knowledge and practical application. It empowers scientists and students alike to predict the amount of product they should expect from a reaction under ideal conditions, making it a critical tool for planning and executing chemical reactions with efficiency.
While the steps and considerations outlined above offer a roadmap to mastering this concept, patience and practice are essential, as is an understanding that actual yields often differ from theoretical predictions because of various uncontrolled factors in the laboratory or industrial settings. Nonetheless, the skill of calculating theoretical yield is invaluable in the field of chemistry.
Frequently Asked Questions
Q: What is the difference between theoretical yield and actual yield?
A: Theoretical yield is the maximum amount of product calculated under ideal conditions based on stoichiometry, while actual yield is the amount of product actually obtained from an experiment or industrial process.
Q: Why is my actual yield lower than the theoretical yield?
A: Actual yield may be lower due to loss of product during the process, unreacted impurities, side reactions, or inefficiencies in the reaction conditions (like temperature and pressure).
Q: Can the theoretical yield ever be greater than 100%?
A: No, theoretical yield represents the maximum possible amount of product, which would be 100% efficiency. Any yield greater than the theoretical value indicates either an error in calculation or unexpected reaction conditions, such as contamination. Actual yield can exceed theoretical yield if the product contains impurities that increase its weight.