Valence electrons are the outermost electrons of an atom and play a pivotal role in chemical reactions and bonding. Understanding how to calculate the number of valence electrons in an atom is fundamental to predicting how elements will interact with each other. This knowledge is not only crucial for chemists but also for anyone interested in the basics of science. Let’s unravel the process of determining valence electrons step-by-step, making it accessible for those without a technical background.
Periodic Table Placement
To decipher the count of valence electrons, the periodic table offers the first clues. The table is your map to understanding elements and their properties.
Detailed Steps:
-
Identify the Element: Find the element on the periodic table whose valence electrons you want to calculate.
-
Determine the Group Number: Look at the column that the element is in. The group number (in most cases for main-group elements) can tell you the number of valence electrons. Elements in the same group have the same number of valence electrons.
-
Verify the Type of Element: Check if the element is a transition metal or a main-group element, as transition metals have a different method for determining valence electrons.
Summary:
Using the periodic table is straightforward and reliable for main-group elements. However, it’s not as clear-cut for transition metals and may require additional concepts for accurate determination.
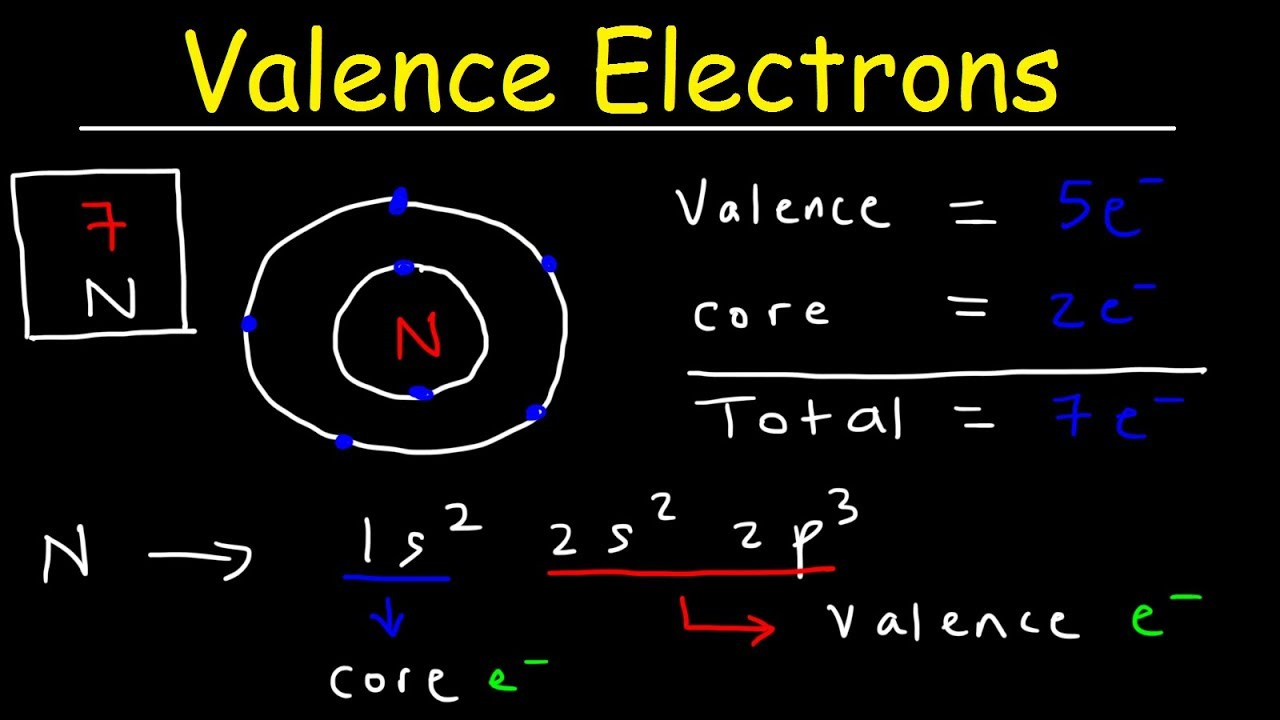
Electron Configuration
The electron configuration of an atom provides a roadmap of where its electrons are situated and how many are in the valence shell.
Detailed Steps:
-
Write the Electron Configuration: Using the periodic table, write out the electron configuration of the atom, layer by layer (1s, 2s, 2p, etc.).
-
Identify the Valence Shell: Find the highest energy level, which contains the outermost electrons.
-
Count the Electrons: Tally the electrons in the valence shell (the outermost energy level) to find the number of valence electrons.
Summary:
Electron configuration gives an accurate valence electron count, including for transition metals. It can be complex for beginners, but it offers a full understanding of an atom’s electronic structure.
Using Group Numbers
For many elements, the group number is a handy indicator of valence electrons.
Detailed Steps:
-
Locate the Element: On the periodic table, find the element in question.
-
Refer to Group Number: Check the element’s group number – for groups 1 and 2 and 13 to 18, this often equals the number of valence electrons.
-
Note Exceptions: Remember that this method doesn’t work for transition metals or lanthanides and actinides.
Summary:
This method suits beginners well but is limited to certain portions of the periodic table and may not always apply due to exceptions.
Valence of Common Elements
Some elements have a consistent number of valence electrons, which can be memorized.
Detailed Steps:
-
Memorize Common Counts: Know that hydrogen and helium have 1 and 2 valence electrons respectively, alkali metals have 1, alkaline earth metals have 2, halogens have 7, and noble gases have 8.
-
Refer to This Knowledge: Use these memorized counts as a quick reference when these elements are involved.
Summary:
Memorization is efficient and fast for familiar elements but doesn’t help with unfamiliar elements or foster deep learning.







