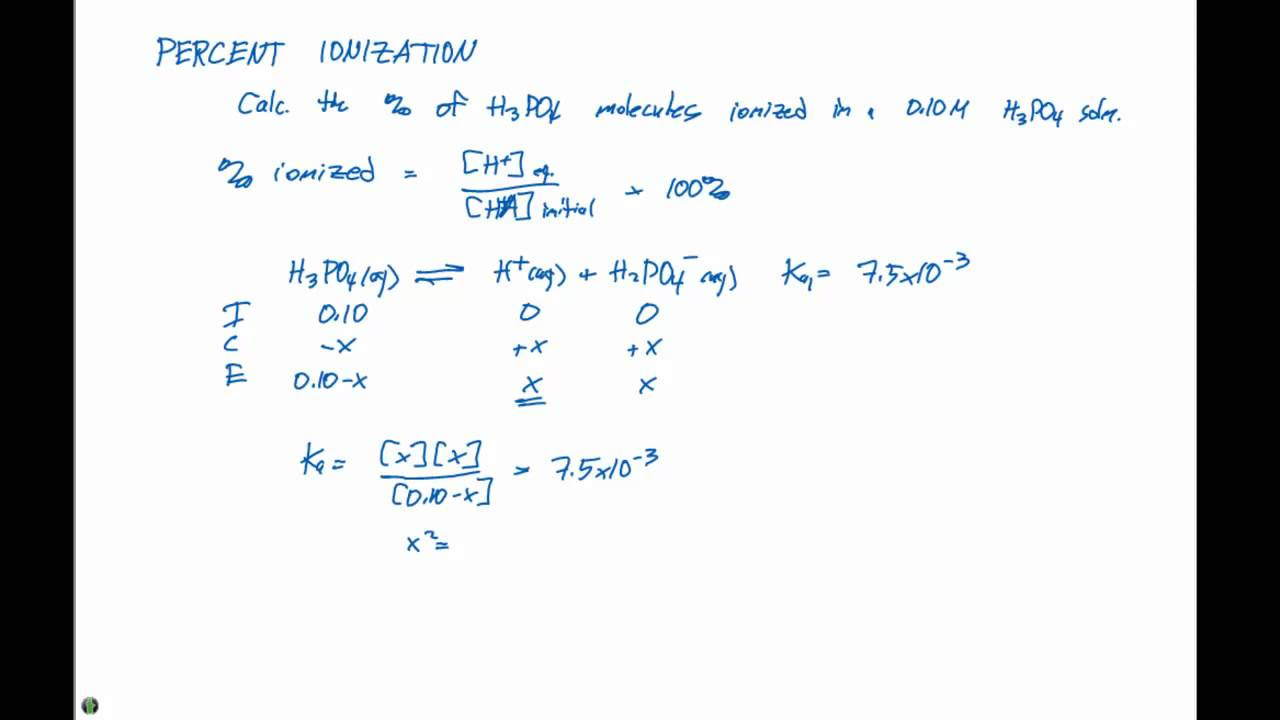
Percent ionization is a key concept in chemistry that describes how much a weak acid or base dissociates into ions in a solution. Understanding this process is important for predicting the behavior of acids and bases in different conditions, which is crucial in many scientific and industrial applications, such as pharmaceuticals, environmental science, and food chemistry. In this guide, we will explore several approaches and tips to calculate percent ionization, making it accessible for even those without a technical background.
Understanding Ionization
Ionization occurs when an acid or base in water breaks down into ions. A weak acid or base doesn’t fully dissociate, so the percent ionization measures the extent of this process. This is a critical parameter used in solution chemistry to predict the pH of solutions, understand buffer systems, and gauge chemical reactivity.
Steps:
- Know the Acid or Base Concentration: Begin by finding the concentration of the acid or base in moles per liter (M).
- Find the pH or pOH of the Solution: Use a pH meter or litmus paper to determine the pH (for acids) or pOH (for bases) of your solution.
- Calculate the [H3O+] or [OH-]: For acids, use the formula [H3O+] = 10^(-pH) to find the concentration of hydronium ions. For bases, [OH-] = 10^(-pOH).
- Determine the Ionized Portion: Use the equilibrium expression relevant to your acid or base to find the ionized portion.
- Calculate Percent Ionization: Take the ionized portion over the initial concentration and multiply by 100 to get the percent ionization.
Summary:
Understanding the ionization of acids and bases through these steps is fundamental in chemistry. While this approach requires some basic mathematical operations, it provides accurate results. A potential downside is the necessity for precise measurements and calculations, which can be challenging without proper equipment or attention to detail.
The Role of the Equilibrium Constant
The equilibrium constant (Ka for acids, Kb for bases) relates to the extent of ionization. A higher constant means a greater degree of ionization. It is a fixed value for each acid or base at a given temperature.
Steps:
- Learn the Equilibrium Constant: Look up the Ka or Kb from a reliable chemical database.
- Set Up the Equilibrium Expression: Write the expression for the dissociation of the acid or base and include the equilibrium constant.
- Use Initial Concentrations: Insert the initial concentration of the acid or base into the equilibrium expression.
- Solve for Ionized Concentration: Rearrange the equation to solve for the ionized concentration.
- Find Percent Ionization: Divide the ionized concentration by the initial concentration and multiply by 100.
Summary:
Using the equilibrium constant offers a precise understanding of ionization levels. It’s great for theoretical calculations but relies on the availability of equilibrium constant values and can be complex for those unfamiliar with equilibrium chemistry.
The Impact of Dilution
Dilution can significantly affect the percent ionization of a weak acid or base, generally increasing it as the solution becomes more dilute.
Steps:
- Dilute the Solution: Mix the acid or base with a known quantity of solvent, usually water, to achieve a specific concentration.
- Recalculate the pH or pOH: Measure the pH or pOH of the diluted solution.
- Adjust the Equilibrium Expression: Reflect the new concentration in the equilibrium expression.
- Solve for New Ionized Concentration: Determine the ionized concentration after dilution.
- Compute the New Percent Ionization: Calculate the percent ionization post-dilution.
Summary:
Dilution is a practical consideration when assessing ionization, especially in laboratory or industrial processes. This method highlights the dynamic nature of ionization but requires careful volume measurements and could potentially dilute the solution beyond usable concentrations.
The Temperature Effect
Temperature changes can shift the equilibrium of a reaction, altering the percent ionization of the substances involved.
Steps:
- Identify the Initial Temperature: Note the starting temperature of your solution.
- Adjust Temperature: Heat or cool the solution to the desired temperature carefully.
- Measure pH or pOH: Determine the new pH or pOH at this temperature.
- Update the Equilibrium Constant: Recognize that Ka or Kb may change with temperature.
- Recalculate Percent Ionization: Perform the percent ionization calculation considering the temperature change.
Summary:
Acknowledging temperature’s role is essential for understanding ionization in different environments. This can lead to a more nuanced picture of how substances will behave but introduces complexity due to the temperature dependence of the equilibrium constants.
Buffer Solutions
Buffer solutions resist changes in pH upon the addition of small amounts of acids or bases, affecting percent ionization calculations.
Steps:
- Identify the Buffer System: Determine the components of the buffer solution.
- Consider the Buffer Capacity: Understand how much the buffer can resist pH changes.
- Factor in the Buffer Components: Incorporate the concentrations of the buffer components into the equilibrium expression.
- Find the Effect on Ionization: Calculate how the buffer alters the ionized concentration.
- Compute Percent Ionization with Buffer: Determine the percent ionization in the buffer’s presence.
Summary:
Buffers are a critical factor in many chemical processes, providing stability against pH changes. For accurate percent ionization in buffers, a deeper knowledge of the buffer components is required, creating a more challenging calculation scenario.
Using a Calibration Curve
A calibration curve can be used to determine percent ionization by comparing known concentrations and pH values to those of the unknown solution.
Steps:
- Prepare Standard Solutions: Create a series of standard solutions with known concentrations.
- Measure Standard pH Values: Analyze the pH values of these standard solutions.
- Create the Calibration Curve: Plot the standard concentrations against their corresponding pH values.
- Use the Curve: Find the pH of your unknown solution and use the curve to determine its concentration.
- Calculate Percent Ionization: Apply the known concentration and measured pH to calculate percent ionization.
Summary:
This method provides a practical and visual way of determining percent ionization, especially useful for solutions with difficult-to-measure concentrations. However, it requires accurate initial standards and can be less reliable if the calibration curve is not well constructed.
Approximation Methods
In some cases, it is possible to approximate the percent ionization using assumptions about the relative sizes of concentrations.
Steps:
- Estimate Concentrations: Make simplified assumptions about the ionization, like assuming that the ionized concentration is much smaller than the initial concentration.
- Apply the Assumption: Use this approximation to simplify the equilibrium expressions.
- Calculate pH or pOH: Estimate the pH or pOH based on these simplified expressions.
- Determine Percent Ionization: Use the estimated values to calculate a rough percent ionization.
Summary:
Approximation makes the percent ionization calculation more accessible for those without advanced chemistry knowledge. Still, it is less precise than other methods and best suited for cases where a rough estimate is sufficient.
Utilizing Online Calculators
There are online tools available that can calculate percent ionization with user input, reducing the need for complex calculations.
Steps:
- Input Data: Enter the known values, like concentration and pH, into the calculator.
- Process the Calculation: Let the calculator apply the formulas and constants.
- Review the Results: Analyze the provided percent ionization.
- Cross-Verify: Optionally compare the results with manual calculations for accuracy.
Summary:
Online calculators simplify the process significantly and quickly provide results. However, blindly relying on these tools can lead to misunderstandings if the input data is not accurate or if there’s an error in the calculator’s programming.
Reviewing Case Studies
Looking at case studies of percent ionization can solidify understanding and provide context for real-world applications.
Steps:
- Select Relevant Case Studies: Choose examples that are relevant to your needs.
- Analyze the Details: Study the methods and data used in the case studies.
- Apply the Principles: Understand how the principles of percent ionization are applied in these scenarios.
- Learn from the Outcomes: Gather insights based on the reported results of these case studies.
Summary:
Case studies offer valuable learning experiences and showcase practical applications, allowing for a deeper comprehension without performing complex calculations. However, it is important to remember that case studies are specific to their scenarios and might not be universally applicable.
Incorporating Experimental Data
Laboratory experiments can provide empirical data that enhance the accuracy of percent ionization calculations.
Steps:
- Design the Experiment: Craft a procedure to measure the ionization indirectly or directly.
- Conduct the Experiment: Carefully execute the experiment, recording all data.
- Interpret the Results: Use the data to determine the actual percent ionization.
- Refine the Theory: Adjust theoretical models based on experimental findings.
Summary:
Experimental data yields highly accurate and tailored results, but carrying out experiments requires laboratory skills and access to specialized equipment, which might not be feasible for all.
Tips and Tricks for Accurate Measurements
- Use fresh reagents: Ensure chemicals are not expired for reliable results.
- Calibrate equipment: Regularly calibrate pH meters and other measuring devices.
- Control variables: Keep conditions like temperature constant during measurements.
Conclusion:
Calculating percent ionization might seem daunting at first, but with the right methods and guidance, it becomes an accessible task. By carefully following the described steps and taking into account factors like equilibrium constants, dilution, and temperature, even those without a technical background can accurately determine percent ionization for various chemical solutions. It’s important to remember that practice and patience are key, as these calculations can be delicate and require attention to detail.
FAQs:
-
What is percent ionization?
Percent ionization is the measure of the extent to which an acid or base dissociates into ions in a solution. -
Why is the equilibrium constant important for calculating percent ionization?
The equilibrium constant represents the degree of ionization of an acid or base at a given temperature, serving as a crucial part of the calculation. -
Can percent ionization be affected by external factors?
Yes, percent ionization can be influenced by factors such as concentration, temperature, the presence of a buffer, and dilution of the solution.