In the core of every atom, an incredibly tiny and intriguing world exists. Here, protons and neutrons form a dense cluster known as the nucleus. While the number of protons defines the element, the neutrons play a critical role in the stability of the atom. Calculating the number of neutrons in an atom is an essential skill in understanding the very building blocks of our universe. Whether you’re a student tackling chemistry homework or a curious mind exploring atomic structure, this guide is designed to help you master the art of neutron calculation with ease.
Understanding Atomic Structure
Before we dive into specific methods for finding the number of neutrons in an atom, it’s crucial to have a basic understanding of atomic structure. An atom consists of three primary particles: protons, neutrons, and electrons. The protons and neutrons form the nucleus, while electrons orbit around the nucleus.
Detailed Steps:
-
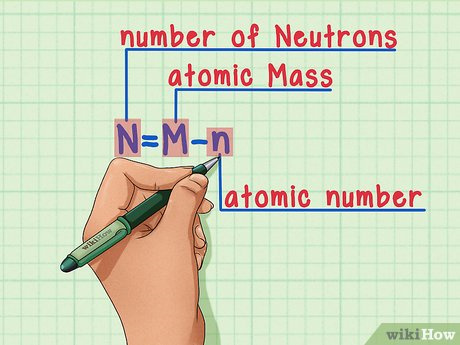
Identify the Atomic Number (Z): The atomic number of an element, usually denoted by the symbol Z, is equal to the number of protons in the nucleus of the atom and can be found on the periodic table.
-
Find the Atomic Mass (A): Atomic mass, or atomic weight, approximates the total number of protons and neutrons in the atom. This number is also listed on the periodic table for each element close to the atomic symbol.
-
Calculate the Number of Neutrons (N): Since N = A – Z, subtract the atomic number from the atomic mass to get the number of neutrons.
Summary:
This method provides a straightforward approach to determining the number of neutrons in an atom and builds a foundational understanding of atomic structure. However, one potential downside is the confusion that may arise from the fact that the atomic mass is often not a whole number because it’s an average of all isotopes.
Utilizing Round Numbers
For many common elements, the atomic mass is close to a whole number despite being an average of isotopes. We can use this to our advantage for a quick estimation by rounding the atomic mass to the nearest whole number.
Detailed Steps:
-
Round the Atomic Mass: Look at the atomic mass on the periodic table and round it to the nearest whole number to simplify your calculation.
-
Subtract the Atomic Number: After rounding, subtract the atomic number from this rounded atomic mass.
Summary:
This is a quick and easy method that’s especially helpful for speedy calculations or estimates. The main downside is that it may result in slight inaccuracies for elements with atomic masses far from whole numbers due to the prevalence of isotopes with different numbers of neutrons.
For Isotopes
Each element can come in different forms called isotopes, which have the same number of protons but a different number of neutrons. When calculating neutrons for isotopes, we must take the specific isotope’s mass number into account.
Detailed Steps:
-
Identify the Isotope: Determine the isotope you’re dealing with, which is typically denoted with the element’s symbol followed by the mass number (e.g., Carbon-14).
-
Use the Mass Number: The mass number of an isotope, which is a whole number, represents the sum of protons and neutrons.
-
Calculate Neutrons: Subtract the number of protons (the atomic number) from the mass number of the isotope to find the number of neutrons.
Summary:
This method is accurate for determining the number of neutrons in a specific isotope. The downside is that you need to know the isotope’s mass number beforehand, which may not always be readily available.
Using a Periodic Table
The periodic table is an invaluable tool in the world of chemistry, containing a wealth of information about the elements, including the data needed to calculate the number of neutrons in an atom.
Detailed Steps:
-
Locate the Element: Find the element on the periodic table.
-
Gather Atomic Number and Mass: Note down the atomic number and atomic mass from the element’s box on the periodic table.
-
Perform the Calculation: Use the formula N = A – Z to calculate the number of neutrons.
Summary:
The periodic table method is both educative and practical. The potential downside is the need to have a periodic table on hand, but since it’s a standard tool in chemistry, this is rarely an actual impediment.
Using Online Calculators
The internet has a variety of tools that can automate the process of calculating the number of neutrons in an atom, taking out the manual computation and reducing the chance for error.
Detailed Steps:
-
Find an Online Neutron Calculator: Search for a reliable online calculator that is designed to compute the number of neutrons in an atom.
-
Input Data: Enter the atomic number and the atomic mass into the calculator.
-
Get Results: Submit the data to receive the number of neutrons.
Summary:
Online calculators offer a high level of convenience and precision. The downside is that relying on calculators can hinder learning and understanding of the underlying concepts.
Through Chemical Formulas
In chemical formulas, isotopes are sometimes indicated, which can directly tell you the number of neutrons if you know how to interpret the information.
Detailed Steps:
-
Analyze the Chemical Formula: Locate any isotope notation in the chemical formula.
-
Determine Mass Number: The mass number is often part of the isotope’s name or symbol in the formula.
-
Calculate Neutrons: Subtract the atomic number from the mass number.
Summary:
Chemical formulas can provide precise isotope information, which is great for specific calculations. However, this method assumes that the user can interpret chemical notation and may not apply to standard element forms without isotope information.
Learning Nuclear Symbols
Nuclear symbols are another way isotopes are represented, where both the atomic number and mass number are written alongside the chemical symbol of the element.
Detailed Steps:
-
Understand Nuclear Symbol: Examine the nuclear symbol, which typically looks like X-A-Z, where X is the chemical symbol, A is the mass number, and Z is the atomic number.
-
Find Neutron Number: Use the mass number (A) and atomic number (Z) from the symbol to calculate the total number of neutrons.
Summary:
This method involves a deeper understanding of nuclear chemistry and can be accurate for finding neutrons in isotopes. One downside is it might be confusing if you’re unfamiliar with nuclear symbols.
Tips and Tricks for Precision
When seeking precision in your neutron calculations, always use the most accurate atomic masses available, preferably from a recent periodic table or authoritative chemistry resources.
Summary:
Precision is key in scientific calculations, but remember that the atomic mass on the periodic table may still have slight variances due to the presence of isotopes.
Understanding Real-World Applications
Knowing how to calculate neutrons has real-world applications, such as in materials science, nuclear physics, and even medicine. This knowledge can deepen your appreciation of the atom’s role in the universe.
Summary:
The ability to calculate the number of neutrons deepens your scientific literacy and equips you with the tools to understand a wide range of scientific phenomena.
Practice Makes Perfect
As with any skill, practice enhances competency. Use exercises and quizzes to reinforce your neutron calculation abilities.
Summary:
Regular practice will help solidify your understanding of the neutron calculation process, leading to quicker and more accurate computations.
Learning how to calculate the number of neutrons can seem daunting at first, but like any science concept, it becomes clearer with explanation and practice. This guide aimed to simplify the process and provide you with several methods and helpful tips to become proficient in finding neutron numbers. Whether for academic purposes or personal interest, mastering this fundamental aspect of atomic science is both rewarding and intellectually stimulating.
Conclusion
Congratulations on learning how to calculate the number of neutrons in an atom! With this knowledge, you’ve taken a leap into the world of atomic science and unlocked a deeper understanding of the matter that composes our universe. Remember, each method has its purpose and context, so use the appropriate one for your needs. Happy calculating!
FAQs
1. Why is the atomic mass not always a whole number?
The atomic mass listed on the periodic table is an average of the masses of all the isotopes of that element, each with its own number of neutrons, which is why it’s not always a whole number.
2. Can you determine the number of neutrons from the chemical symbol alone?
No, the chemical symbol does not provide enough information on its own. You need at least the atomic number or the mass number along with the chemical symbol to calculate the number of neutrons.
3. How do isotopes affect the calculation of neutrons?
Isotopes have the same number of protons (atomic number) but different numbers of neutrons. To calculate neutrons in a specific isotope, you need to know the mass number of that isotope, which is unique to each isotope of an element.








